
Rune Labs, a precision neurology firm in San Francisco, announced that its StrivePD software program ecosystem for Parkinson’s illness has been granted 510(okay) clearance by the U.S. Food and Drug Administration (FDA) to start utilizing the Apple Watch to gather and measure information from Parkinson’s sufferers.
The FDA approval is one more reason the Apple Watch is an enormous participant in serving to folks with Parkinson’s. While there are a number of medical-grade gadgets able to monitoring Parkinson’s signs, many customers will need an Apple Watch as a result of it’s acquainted to them and has different makes use of — like health monitoring, coronary heart price monitoring and, most just lately, remedy monitoring, amongst different issues.
The StrivePD software program makes use of Apple’s Movement Disorder API (Application Programming Interface) to trace tremors and dyskinetic signs of Parkinson’s from the Apple Watch. The movement information is collected in an iPhone app, permitting sufferers to take notes about their signs, total temper, remedy utilization and extra.
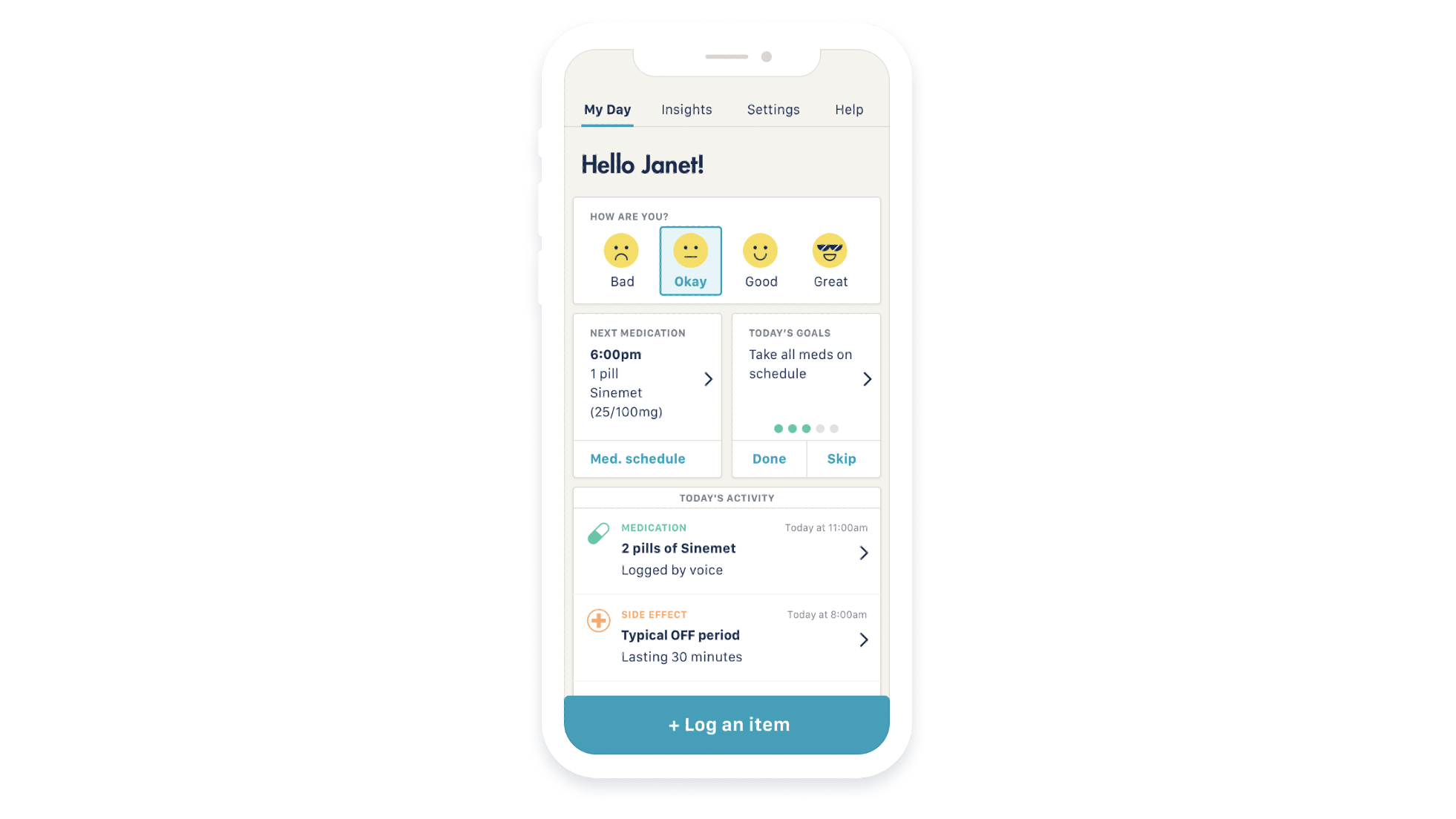
Image Credits: Rune Labs
Other firms, corresponding to Cerebellia, are additionally making use of Apple’s Movement Disorder API to offer Parkinson’s sufferers related data. However, the FDA clearance implies that the StrivePD app is the primary vital use of Apple’s software program instruments for measuring motion issues since Apple released the features in 2018.
Aura Oslapas, a Parkinson’s affected person and the developer of the unique StrivePD app, said in a statement, “When people with Parkinson’s are prescribed new medications, adjusting how much to take and when to take it until they find something that works can be a lengthy process. StrivePD helps people track their symptoms and improvements, accelerating the time to an optimal medication schedule — and with today’s clearance, more people will have access to this life-changing technology.”
“StrivePD on Apple Watch is the long-awaited union of quantitative and qualitative data that encourages better care and communication between patients and clinicians while also empowering people with Parkinson’s who are striving to live better every day,” Oslapas stated.
Rune Labs’ purpose is to make use of mixed information — from the Apple Watch, Rune Labs’ StrivePD software program, and knowledge from different sources, like a Medtronic implant to measure mind indicators — to tell a health care provider’s selections on how you can deal with a affected person.
This can also be a big milestone for the startup because it seeks to develop its work with pharma and Medtech firms. Without the superior expertise, medical doctors can solely collect information a couple of Parkinson’s affected person’s actions by observing them throughout a brief scientific go to. It’s additionally troublesome for a affected person affected by a neurological dysfunction to watch each involuntary motion at residence with no machine serving to them.
“As we have seen in oncology, the introduction of large quantities of real-world data has the power to transform drug development and fundamentally change disease prognosis. This clearance is a major step towards building a similar paradigm in neurology,” Brian Pepin, CEO of Rune Labs, added. “With all of the data we will collect and the patients we will reach through this clearance, we will make sure the right participants enroll in trials and help our pharma and Medtech partners run more efficient trials with higher quality outcomes data, thereby enabling more therapies to come to market quickly to help those suffering from Parkinson’s.”
Rune Labs instructed TechCrunch that the software program had been utilized by sufferers on the University of California at San Francisco for a yr and Mount Sinai for six months. The FDA approval means clinicians now have methods to invoice sufferers when reviewing information and lets trial sponsors use the findings for research, the corporate stated.
Apple has internally explored how you can use the Apple Watch and iPhone to watch Parkinson’s signs in addition to filed for a patent application for extra superior expertise to deal with or diagnose the illness. In 2021, Apple researchers printed data demonstrating that the watch might precisely monitor modifications within the motor signs of Parkinson’s sufferers.
While the Cupertino tech large shouldn’t be formally concerned with the Rune Labs mission, Rune Labs spokesperson Elizabeth Eaton instructed The Verge that Apple up to date its software program to match FDA necessities and wrote a letter of help as a part of Rune Labs’ FDA utility. However, Eaton instructed TechCrunch that Rune “cannot comment on the Apple Watch software requirements, and cannot comment further on Apple’s involvement.”
#FDA #clears #Apple #Watchbased #Rune #Labs #Parkinsons #tracker #TechCrunch
Brain data startup Rune Labs gets FDA clearance for Apple Watch-based Parkinson’s tracker